Oral Therapeutics for Chronic Fatigue
Treating Patients with Exertional Intolerance and Post-Exertional Malaise
Mitodicure is developing oral therapeutics to address significant unmet medical needs with an initial focus on ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome).
ME/CFS is a very debilitating disease. Exertional intolerance and post-exertional malaise are the cardinal symptoms. ME/CFS can develop after a viral infection or physical/mental overexertion. 6 million people are affected in 7 countries alone (U.S., UK, Germany, France, Italy, Spain, Japan). There are four to five times more patients than with Multiple Sclerosis with a comparably poor quality of life. 25% of ME/CFS patients are bedbound and helpless. Almost 70% are without a job. Since ME/CFS is also one of the most dramatic forms of Long COVID, its occurrence is on a strong rise. Available treatments only address the symptoms. There are no curative treatments. No drug has ever been approved for ME/CFS.
Our lead program, MDC002, is a novel oral treatment being developed to treat all people living with exertional intolerance and post-exertional malaise for the first time.
The vicious circle in which patients are trapped will be interrupted
ME/CFS patients only have enough muscle energy to maintain bodily functions for simple survival. Muscle cell necrosis and mitochondrial damage in skeletal muscle are proven.
Mitodicure’s pharmacological strategy is directed against the pathomechanisms causing exertional intolerance and post-exertional malaise. Both are due to an energy deficit caused by ionic disturbances, mitochondrial dysfunction, and hypoperfusion which can be remedied by MDC002 stimulating the sodium-potassium pump Na+/K+-ATPase and the mitochondrial sodium-calcium exchanger NCLX in skeletal muscle. Furthermore, MDC002 also improves muscle/brain perfusion, edema, and pain. As a result, the muscle cells and their damaged pool of mitochondria can regenerate. The previously harmful mitochondrial fission caused by the exercise-induced high calcium level is inhibited, but the natural fission process remains intact for mitochondrial division and recovery. Patients will get back their energy.
MDC002 Breaks the Vicious Circle at Several Synergistic Points
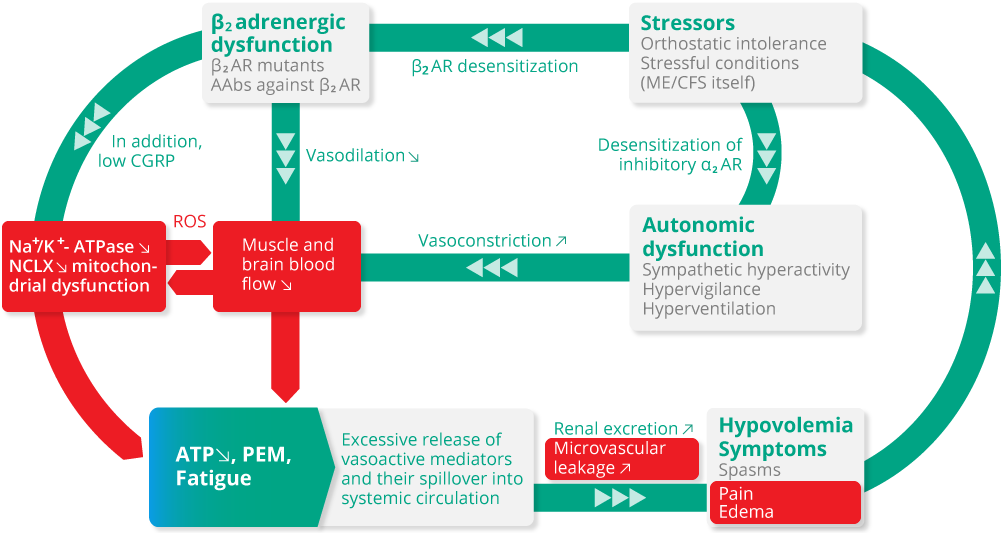
ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) is an acquired mitochondrial myopathy that also leads to vascular dysfunction via reactive oxygen species. Potential risk factors for the disease are autoantibodies, collagen diseases, and variants in mitochondrial, vascular, and muscle genes. Once fully developed, mitochondrial dysfunction, caused by sodium-induced calcium overload in skeletal muscle, reproduces itself with every post-exertional malaise (PEM) keeping ME/CFS patients captured in a vicious circle from which they cannot escape. MDC002 is being developed to break this vicious circle.
Red areas: These bad processes are interrupted and so the vicious circle dissolves.
AAbs: Autoantibodies; AR: Adrenergic receptor; ATP: Adenosine triphosphate; CGRP: Calcitonin gene-related peptide; Na+/K+-ATPase: Sodium-potassium pump; NCLX: Mitochondrial sodium-calcium exchanger; PEM: Post-exertional malaise; ROS: Reactive oxygen species.
